Which of the Following Has the Lowest Ph
Its pH is reported to be 26 to 27 mainly due to H 3 PO 4 phosphoric acid. PH will be maximum.
Which of the following enzymes has the lowest pH optimum.
. A A solution with a pH of 340 will have a higher concentration of hydroxide ions than hydronium ions. Which of the following body fluids has the lowest pH. So this salt is a basic salt.
Among HF HCl and HBr Hydrofluoric acid HF is the weak acid. Hence HCN has the lowest Ka and the lowest hydrogen. 01 M HBO pka 243 01 MHA pka 455 01 M HMO pKa 823 01 M HST Pka 1189 pure water O HA HST HMO HBO pure water Question 25 Select the correct name for the following compound.
You may get confused by the pH range. Which of the following solutions would have the lowest pH. Which of the following solutions would have the lowest pH.
Thus has highest pH. The Ka is called the acid dissociation constantIt shows the extent to which an acid is ionized in waterThe pH shows the hydrogen ion concentration of waterThe higher the Ka the higher the hydrogen ion concentration and the lower the pH. Chemistry questions and answers.
Which of the following buffers has the largest capacity if made in 10 L solutions. Nitric acid is the strongest acid and it has lowest pH. Which of the following solutions has the lowest pH at 25 o C.
Low pH means highest acidity and highest acidity means the one with the largest Ka which means the strongest acid. The pH scale ranges from 0-14 pH. Gastric juice A _____ is a group of similar cells that perform similar functions.
It has the highest pH ie towards 7 but less than 7. D A solution with a pOH of 1320 is very basic. A 01 M NaCN B KCl C NH4Cl D NaOH please explain how to solve this thanks.
17 Which of the following solutions has the lowest pH at 25 o C. Which of the following substances has the lowest pH. Difference between pH of 4 and pH of 10.
You can predict that HPO4 2- is less strong because the third dissociation is weaker the first. You can discard easily the options B and C because they are organic acid which are relatively weak. Sulfuric acid has a pH of 03.
The stronger the acid the lower the pH because pH log 1 H. 0300 mol HCN. This is a salt formed by the reaction of strong base and weak acid.
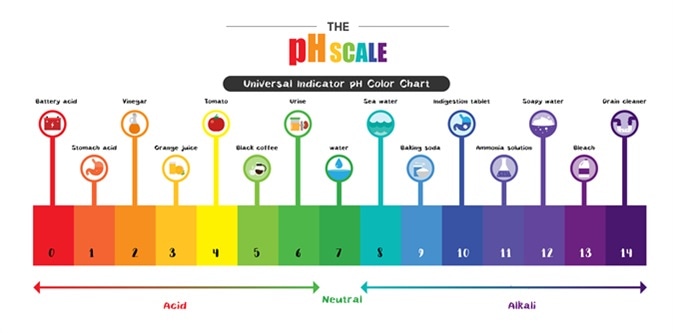
B A solution with a pOH of 210 is very acidic. Which acid has the lowest pH. From all the above options vinegar has the lowest pH-value that means it is the most acidic from the above and the washing soda is most basic.
We know that pH -log H pH. Which of the following 10 M solutions would have the lowest pH. Which of the following could be XBr.
To compare properly the inorganic acids you should use a Ka or pKa table. Which of the following organs is an accessory organ of the digestive system. 1 Nitric acid HNO3 is the strong acid.
Question 24 Which of the following acids has the lowest pH. онанснунанснан CH2CH3 ortho-ethylheptylcyclopentane meto. What is the lowest pH acids on a pH scale.
BUT you can reason which will have the lowest pH. 72E-4 x x 1-x Solve for x 00268 M H and pH 157. No calculations required A 02 M sodium hydroxide B 02 M hypochlorous acid C 02 M.
HCN has the highest pH among all the acids listed in the question. No calculations required a 02 M sodium hydroxide b 02 M hypochlorous acid c 02 M ammonia d 02 M benzoic acid e pure water 16. So ultimately the pH of 02N or 02M HNO3 will be the lowest with a value of 06990.
The products of a strong acidstrong base reaction are. A 0200 M solution of XBr has a pH of 267. In a similar manner the pH of the other acids can be calculated then compared to pick the one with the lowest pH.
Which of the following has the lowest pH. C A solution with a pH of 820 is only slightly basic. How acidic is Coke.
Correct option is C As the H concentration will be least in case of CaOH 2. The pH of aqueous solution of ammonium formate is pKa of HCOOH 38 and pKb of NH3 48 asked Jan 6 2019 in Equilibrium by Sahida 800k points equilibrium. Hence the correct option is b- vinegar.
Which of the following aqueous solutions will have highest pH a NaCl B ch3coona C Na2CO3. It should be noted that the lesser the pH value the stronger is the acid and the higher the pH-value the stronger is the base. A 010 M solution of a weak acid HX is.
What is Cokes pH level. The Acidity of the given acids will be in the order 02M HNO3 01M H2SO4 01M HCl 02M CH3-COOH.



No comments for "Which of the Following Has the Lowest Ph"
Post a Comment